Regulatory Standards for Aseptic Records
Jan 14, 2026
Aseptic records are critical for ensuring compliance and maintaining product safety in sterile compounding. Whether you're operating a 503A pharmacy or a 503B outsourcing facility, precise documentation is non-negotiable. Here's what you need to know:
503A Pharmacies: Governed by the revised USP <797> (effective November 1, 2023), focusing on patient-specific prescriptions and category-based environmental monitoring.
503B Facilities: Regulated under FDA's cGMP standards (21 CFR 210/211), requiring stricter controls like daily monitoring and independent quality oversight.
Key Documentation: Includes personnel training, environmental monitoring, sterility testing, and operational logs for temperature, pressure, and cleaning activities.
Differences: 503B facilities face more rigorous standards, such as batch sterility testing, validated expiration dates, and formal investigations for issues.
Both types of facilities must prioritize thorough recordkeeping to meet regulatory expectations and protect patient safety. Below, we break down the specific requirements and best practices for each.
USP <797> Documentation Requirements for Aseptic Technique
Purpose of USP <797> in Aseptic Recordkeeping
The revised USP <797> (effective November 1, 2023) sets mandatory standards for 503A pharmacies, ensuring sterile compounding practices meet strict guidelines. Documentation plays a critical role in proving compliance during inspections, acting as evidence that proper procedures are being followed.
The goal of USP <797> is to minimize risks that could harm patients, such as contamination, sub-potency, or super-potency. Accurate recordkeeping helps identify potential issues early, preventing adverse outcomes. Key areas of focus include staff training on garbing, hand hygiene, maintaining aseptic technique in an ISO Class 5 environment, and disinfecting gloves. These measures are essential to producing sterile preparations free from microorganisms and pyrogens.
Below, we outline the essential documentation elements required for USP <797> compliance.
Required Documentation Elements in USP <797>
Personnel competency records: These records must detail initial training and routine re-evaluations for tasks like garbing, hand washing, aseptic technique in ISO Class 5 environments, and glove disinfection. For initial garbing, three successive successes are required. Ongoing assessments include every 6 months for Category 1/2 or every 3 months for Category 3 compounded sterile preparations (CSPs) for media fills and gloved fingertip testing.
Environmental monitoring logs: These logs capture data on both viable (microbial) and non-viable (particulate) air sampling. An ISO Class 5 environment must not exceed 3,520 particles (≥0.5 µm) per cubic meter. Buffer areas require at least 30 air changes per hour, while hazardous drug storage areas need a minimum of 12 air changes per hour to effectively manage airborne contaminants. For viable air sampling, frequency is every 6 months for Category 1/2 or monthly for Category 3; surface sampling is monthly for Category 1/2 or weekly for Category 3. Non-viable particles must be monitored daily in ISO 5 during operations. Incubation occurs at dual temperatures (30-35°C for 7+ days, then 20-25°C for 7+ days).
Facility maintenance logs: Cleaning and disinfection activities must be thoroughly documented. USP <797> highlights environmental contact as a major source of microbial contamination, emphasizing the importance of meticulous cleaning. Logs should include the date, time, and details of disinfectants used, such as sterile 70% isopropyl alcohol.
Equipment certification records: These records verify that Primary Engineering Controls (PECs), such as laminar airflow workbenches, are certified every six months. HEPA filters in these PECs must meet a 99.99% efficiency standard for removing particles as small as 0.3 microns.
Operational parameter logs: These logs monitor key conditions like pressure differentials, temperature, and humidity. For buffer and ante-areas that are physically separated, the required pressure differential is 0.020–0.030 inch water column. Temperature should be kept at or below 68°F to prevent sweating, which can increase particulate shedding. Relative humidity must always remain under 60%.
FDA cGMP Standards for 503B Recordkeeping under 21 CFR 210/211
Overview of 21 CFR 210/211 for 503B Facilities
503B outsourcing facilities are required to comply with cGMP regulations as outlined in 21 CFR 210/211. According to section 501(a)(2)(B) of the FD&C Act, these facilities must adhere to these standards until more specific regulations are introduced.
At the core of cGMP compliance is the establishment of an independent Quality Control Unit (QCU). This unit is strictly focused on oversight and cannot be involved in production activities. The QCU is responsible for approving all components, processes, and finished products and for reviewing production records to identify and investigate errors.
"The term 'current good manufacturing practice' includes the implementation of oversight and controls over the manufacture of drugs to ensure quality, including managing the risk of and establishing the safety of raw materials, materials used in the manufacturing of drugs, and finished drug products." - FD&C Act
One of the key responsibilities of the QCU is conducting an annual quality review. This involves evaluating written records at least once a year to ensure quality standards are met and determining whether specifications or manufacturing procedures need adjustments. This proactive approach helps facilities monitor trends and address potential issues before they become significant problems. These requirements emphasize the importance of precise and thorough recordkeeping, which is detailed below.
Required Records under cGMP
To meet cGMP standards, facilities must maintain specific records that demonstrate compliance. These include:
Master Production and Control Records (§ 211.186): These serve as the detailed blueprint for every drug product. They must include the product name, strength, dosage form, a full list of components with quantities, and clear manufacturing instructions. Preparation of these records requires one person to create them and another to independently review and sign off, reducing the risk of errors.
Batch Production and Control Records (§ 211.188): These records document every significant step involved in producing each batch. Information such as component weights, in-process results, and personnel involved must be included, along with a specimen of all labels.
Laboratory Records (§ 211.194): These must provide complete data from all tests performed to ensure the product meets specifications. Details include sample descriptions, test methods, raw data like graphs and charts, and calibration records for laboratory instruments.
Equipment Cleaning and Use Logs (§ 211.182): These logs track all cleaning activities, including the date, time, and batch details. Both the person performing the cleaning and a second individual verifying the work must sign these logs.
Record Retention (§ 211.180): Facilities are required to retain production, control, and distribution records for at least one year after the batch's expiration date. These records must be easily accessible for FDA inspections. Additionally, adverse event records must be kept for 10 years, significantly longer than standard production records.
21 CFR Section | Record Type | Key Requirements |
|---|---|---|
§ 211.186 | Master Production Records | Product name/strength, component list, manufacturing instructions; double-checked signatures |
§ 211.188 | Batch Production Records | Documentation of each step, weights used, in-process results, personnel identification |
§ 211.194 | Laboratory Records | Complete test data, methods, raw data (graphs/charts), instrument calibrations |
§ 211.182 | Equipment Logs | Date, time, product, lot number; signatures for cleaning/maintenance |
§ 211.196 | Distribution Records | Consignee name/address, date shipped, quantity, lot number |
If a batch discrepancy or failure occurs, it triggers a mandatory investigation. The outcomes and any follow-up actions must be documented, regardless of whether the batch was distributed. This meticulous recordkeeping is essential for maintaining patient safety and ensuring high standards in aseptic manufacturing practices.
Live Webinar: USP 797 Compliance: A Guide to Proper Donning of Garb for Sterile Compounders
Differences in Aseptic Records Between 503A and 503B Facilities
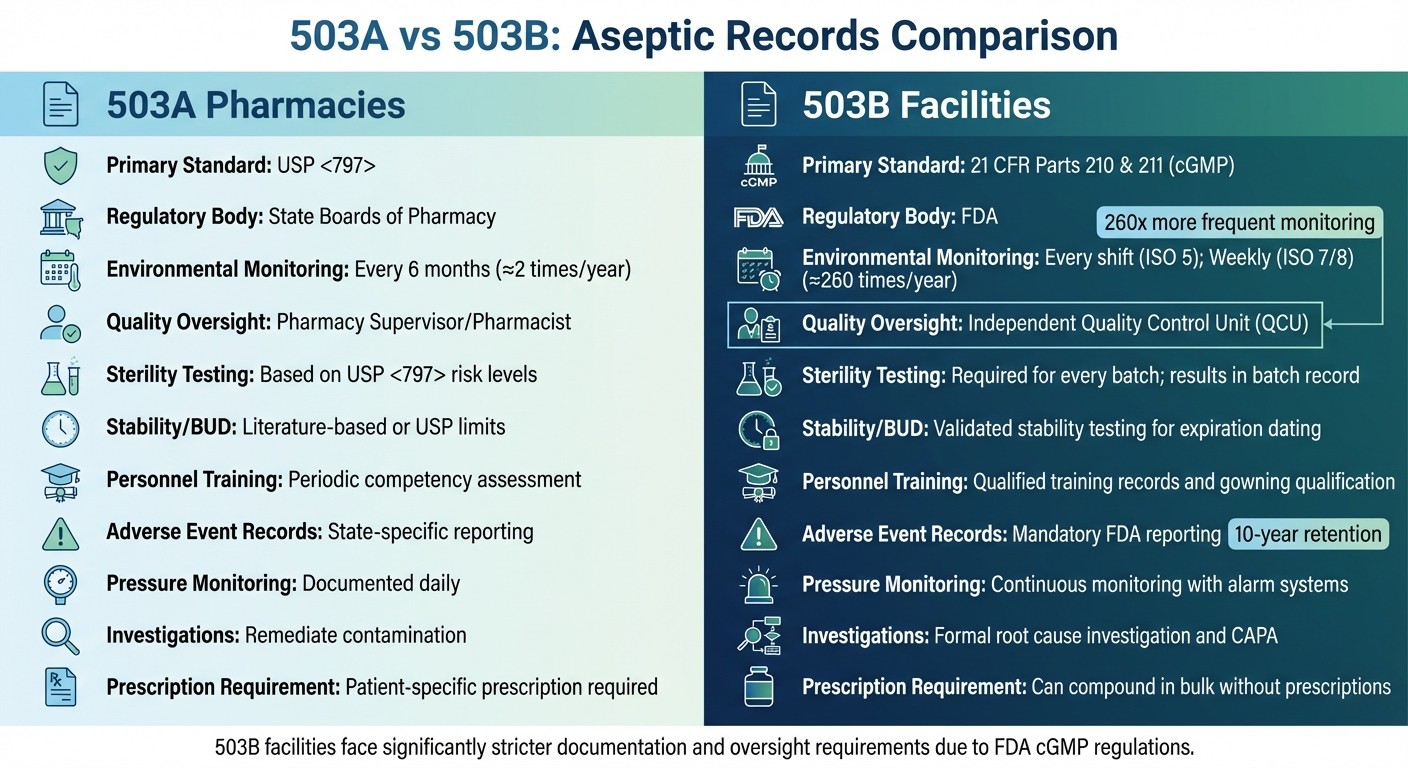
503A vs 503B Aseptic Recordkeeping Requirements Comparison
503A vs 503B Aseptic Recordkeeping Requirements Comparison
The way 503A and 503B facilities handle recordkeeping is shaped by their distinct regulatory requirements. 503A pharmacies operate under the oversight of state boards of pharmacy and adhere to the revised USP <797> standards (effective November 1, 2023). On the other hand, 503B outsourcing facilities are regulated by the FDA and must follow the Current Good Manufacturing Practice (cGMP) guidelines outlined in 21 CFR Parts 210 and 211.
One standout difference lies in how often environmental monitoring is conducted. In 503A pharmacies, viable air sampling is every 6 months for Category 1/2 or monthly for Category 3; surface sampling is monthly for Category 1/2 or weekly for Category 3. Non-viable particles are monitored daily in ISO 5 during operations. However, 503B facilities are required to carry out monitoring at least once per production shift in ISO 5 areas and weekly in ISO 7 and ISO 8 areas. To put this into perspective, a typical 503B facility might conduct around 260 environmental monitoring activities annually, compared to the category-based frequencies for 503A pharmacies.
Another critical distinction involves quality oversight. In 503A pharmacies, quality-related decisions are made by a licensed pharmacist or designated person. Meanwhile, 503B facilities are required to have an independent Quality Control Unit (QCU) with the authority to oversee investigations and approve product releases. The QCU is also tasked with conducting an annual review of all compounding records, including trends in environmental monitoring and media fill results.
"The quality control unit should be independent; that is, the quality control unit should not take on the responsibilities of other units of the outsourcing facility's organization... in order to preserve the integrity of the quality control unit's functions." - FDA Guidance for Industry
503B facilities also have stricter requirements for process validation. They must validate each manufacturing process using multiple batches and conduct stability testing to establish expiration dates. In contrast, 503A pharmacies rely on category-based Beyond Use Dating (BUD) as outlined in USP <797>: Category 1 (e.g., ≤12 hours at room temperature), Category 2 (e.g., up to 90 days with testing), Category 3 (e.g., up to 180 days with enhanced controls).
The table below highlights these and other key differences between 503A and 503B facilities:
Feature | 503A (Revised USP <797>) | 503B (FDA cGMP) |
|---|---|---|
Primary Standard | Revised USP <797> (2023) | 21 CFR Parts 210 & 211 (cGMP) |
Regulatory Body | State Boards of Pharmacy | FDA |
Environmental Monitoring Frequency | Viable air: Every 6 months (Cat 1/2) or monthly (Cat 3); Surface: Monthly (Cat 1/2) or weekly (Cat 3); Non-viable: Daily in ISO 5 | Every shift (ISO 5); Weekly (ISO 7/8) |
Quality Oversight | Pharmacy Supervisor/Pharmacist or Designated Person | Independent Quality Control Unit |
Sterility Testing | Not required for Cat 1; Based on BUD for Cat 2 (e.g., required if >30 days refrigerated); Always for Cat 3 | Required for every batch; results included in the batch record |
Stability/BUD | Category-based (e.g., Cat 1 ≤12 hours RT; Cat 2 up to 90 days; Cat 3 up to 180 days) | Validated stability testing for expiration dating |
Personnel Training | Periodic competency assessment: Every 6 months (Cat 1/2) or 3 months (Cat 3) for media fills/fingertip testing | Qualified training records and gowning qualification |
Adverse Event Records | State-specific reporting | Mandatory FDA reporting |
Pressure Monitoring | Documented daily | Continuous monitoring with alarm systems |
Investigations | Remediate contamination | Formal root cause investigation and CAPA |
Prescription Requirement | Patient-specific prescription required | Can compound in bulk without prescriptions |
These differences underscore the stricter controls and oversight required for 503B facilities, reflecting their broader role in producing medications for bulk distribution.
Records Needed to Prevent Contamination
Thorough documentation plays a key role in identifying and managing potential contamination risks, ensuring sterile conditions are maintained. Beyond meeting regulatory standards, these records serve as a critical safeguard against contamination. Three main types of records - environmental monitoring, personnel competency, and sterility testing - work together to uphold strict aseptic practices.
Environmental Monitoring Records
Environmental monitoring is essential for detecting changes in microbial activity, particle levels, temperature, pressure, and humidity. These records track both non-viable particles (inert particles that may carry microbes) and viable particles (living organisms like bacteria, yeast, and mold).
For non-viable particles, ISO Class 5 areas in 503B facilities must be monitored daily during production. Viable particle monitoring involves active air sampling, passive air sampling (using settle plates), and surface sampling with contact plates or swabs to confirm environmental integrity.
Pressure logs document the positive differential pressure, which should remain between 0.020–0.030 inch water column. Daily temperature and humidity logs ensure appropriate conditions, with relative humidity kept below 60% and temperatures set for worker comfort.
An example of non-compliance highlights the importance of accurate documentation. Between October 2020 and January 2021, a compounding firm received an FDA 483 for failing to record total particle counts in an ISO 5 area during production. Despite this, the firm produced 14 batches and distributed 13 for patient use.
ISO Class | Viable Air Action Level (CFU/m³) | Surface Action Level (CFU/device) |
|---|---|---|
ISO 5 | >1 | >3 |
ISO 7 | >10 | >5 |
ISO 8 | >100 | >50 |
This environmental data feeds directly into personnel assessments, ensuring operators consistently meet aseptic standards.
Personnel Training and Competency Records
Operator performance is another critical factor in contamination prevention. Records like gloved fingertip testing and media fills track whether personnel can maintain aseptic conditions during complex tasks.
For initial training, operators must pass three consecutive gloved fingertip tests with zero CFUs to demonstrate competency. In ISO Class 5 environments, the action level for gloved fingertip sampling after a media fill is more than 3 CFUs for both hands. These records help the Quality Control Unit monitor individual performance and identify staff who may need retraining. Frequencies: Every 6 months for Category 1/2 or 3 months for Category 3.
"Personnel can significantly affect the quality of the environment in which sterile products are processed. A vigilant and responsive program should be established." - FDA Guidance for Industry
A 2019 FDA inspection underscored the importance of accurate competency records. An operator wore two pairs of gloves during fingertip sampling but only one during production, creating a mismatch between testing conditions and actual practice.
Sterility Testing and Adverse Event Documentation
While passing a sterility test is reassuring, it doesn’t guarantee the absence of microbial contamination, which can be unevenly distributed. Sterility testing records are vital for compliance and ongoing quality assurance, reinforcing both regulatory standards and patient safety. Media fill validation records simulate challenging aseptic conditions to ensure sterility. These validations must be performed every 6 months for Category 1 and 2 compounded sterile preparations, and every 3 months for Category 3.
Method suitability records confirm that the product doesn’t inhibit microbial growth, ensuring test accuracy. Additionally, growth promotion testing verifies that each batch of media used in sterility testing or environmental monitoring can support microbial growth.
"Compounding facilities producing purportedly sterile drug products under insanitary conditions should not rely upon or cite a passing sterility test result as an indication of product sterility." - FDA Guidance for Industry
Under Section 503B of the FD&C Act, outsourcing facilities are required to maintain and submit adverse event reports to the FDA. These reports document any patient harm linked to compounded products and are a vital part of the facility’s quality assurance efforts.
How to Improve Aseptic Documentation and Prepare for Audits
Creating Standard Documentation Procedures
To ensure consistency and compliance in aseptic operations, it’s essential to establish written Standard Operating Procedures (SOPs). These should address sanitation practices, cleaning schedules, and methods to prevent microbiological contamination across all processes. For 503B facilities, having an independent Quality Control Unit (QCU) is non-negotiable. This unit must remain separate from production activities to preserve its objectivity and focus on sterility assurance and microbiological quality.
When it comes to batch record reviews, thoroughness is key. These records should include all sampling and testing data, and for sterile products, sterility test results must be incorporated once they’re available. The QCU is also tasked with conducting annual reviews of batch records to identify trends and make necessary adjustments to control measures. Any discrepancies, failures (such as media fill or stability issues), or yield variations must be investigated and documented in detail.
"Quality is best assured by implementing appropriate controls throughout the manufacturing process, with end-product testing providing additional assurance." - FDA
Daily monitoring logs are another critical element. These logs should capture pressure differentials for every shift and record temperature readings. HEPA filters, vital for maintaining sterile environments, must undergo leak testing at least twice a year. Cleaning records should detail schedules, methods, and disinfectant use. Importantly, environmental data should be evaluated individually rather than averaged, as this approach helps pinpoint localized contamination issues.
By implementing these standardized procedures, facilities can build a strong foundation for audit readiness.
Preparing for Regulatory Inspections
To stay audit-ready, facilities must focus on trending data by location, operator, and shift. This helps identify deviations from normal operating conditions early on. FDA 483 observations often highlight gaps in pressure monitoring and active air sampling, underscoring the importance of maintaining accurate, time-stamped records. These records not only meet regulatory standards but also play a crucial role in preventing contamination.
Smoke studies during active operations are another essential practice. Conduct these studies with personnel present and operations underway to verify unidirectional airflow. Record the studies on video to provide evidence during inspections. Non-viable particle counting probes should be positioned within one foot of the work site and aligned with the airflow direction. Personnel records must document initial and annual gowning qualifications, including glove fingertip testing performed in critical areas before glove sanitization.
For instance, during an FDA inspection in March 2019, an operator was observed wearing two pairs of gloves only during sampling to create a false impression of compliance. Additionally, the particle counter was placed at the back of the laminar flow hood, far from aseptic manipulations. Both practices resulted in deficiency observations.
By addressing these issues and maintaining detailed records, facilities can better prepare for inspections and ensure compliance.
Using 503Pharma Educational Resources
503Pharma offers a wealth of educational materials tailored for 503A and 503B compounding pharmacies. Their resources cover best practices for sterile compounding, clean room design, and strategies for building effective quality systems. These tools help pharmacies align with regulatory expectations, streamline documentation processes, and prepare for inspections.
Conclusion
Aseptic records serve as the backbone of compliance and patient safety, capturing every step of production to pinpoint contamination risks before they can affect patients. Even a passing sterility test doesn’t guarantee safety if environmental records show lapses. This is why thorough documentation - covering everything from pressure differentials to personnel monitoring - is absolutely critical.
The level of documentation required varies depending on the type of facility. For 503A pharmacies, compliance aligns with the revised USP <797> (2023) and state regulations. On the other hand, 503B outsourcing facilities operate under stricter federal cGMP standards outlined in 21 CFR 210/211. Both types of facilities face severe penalties for "insanitary conditions", but 503B facilities bear additional responsibilities, including daily environmental monitoring and oversight by an independent Quality Control Unit.
"Compounded medications made without the guidance of standards may be sub-potent, super potent or contaminated, exposing patients to significant risk of adverse events or even death." - USP
Good recordkeeping isn’t just about meeting regulatory demands - it’s about creating a system that actively prevents contamination. Practices like setting alert and action levels, tracking data trends by location and operator, and documenting pressure reversals help build a proactive approach to patient safety.
To support these efforts, resources like those provided by 503Pharma can simplify the process. Their templates and tools are designed to help facilities align USP <797> practices with cGMP standards. By adopting these practical strategies, compounding pharmacies can improve their documentation processes, approach inspections with confidence, and, most importantly, ensure safer medications for the patients who rely on them.
FAQs
What are the key differences in recordkeeping requirements for 503A and 503B pharmacies?
503A pharmacies must keep patient-specific compounding records. These include details tied to USP <795> and <797> standards, such as environmental monitoring and category-based beyond-use dating. This documentation plays a key role in meeting guidelines for personalized patient care.
In contrast, 503B outsourcing facilities operate under CGMP (Current Good Manufacturing Practice) standards. They are required to maintain detailed manufacturing records, including batch production logs, validation documents, and other critical data. These records help ensure the quality and consistency of medications produced on a larger scale.
Why is environmental monitoring required more frequently in 503B facilities than in 503A pharmacies?
Environmental monitoring happens more often in 503B outsourcing facilities because they must adhere to the FDA's Current Good Manufacturing Practices (CGMPs). These regulations demand stricter and more frequent testing of air and surfaces to ensure sterility and maintain rigorous quality standards.
On the other hand, 503A pharmacies operate under the revised USP <797> guidelines (2023), which have category-based monitoring requirements (e.g., viable air every 6 months for Category 1/2 or monthly for Category 3). This is because 503A facilities focus on preparing medications on a smaller scale, tailored for individual patients. The heightened monitoring in 503B facilities aligns with their larger production volumes and the responsibility to deliver safe, high-quality products to a broader patient base.
What do USP <797> and FDA cGMP standards require to ensure sterile and safe compounded medications?
The revised USP <797> (2023) sets critical standards for compounding sterile preparations (CSPs) to safeguard patients from contamination, incorrect potency, and other potential risks. These guidelines stress the importance of having documented procedures, conducting an annual review of standard operating procedures (SOPs), and ensuring comprehensive personnel training. Compounding must take place in carefully controlled environments, such as ISO Class 5 areas, with stringent environmental monitoring, validated cleaning processes, and sterility testing to confirm reliable beyond-use dates based on categories (1, 2, 3).
The FDA’s current Good Manufacturing Practice (cGMP) standards complement these guidelines by requiring pharmacies to maintain a quality-focused environment. This includes constant monitoring of microbial counts, air quality, temperature, and pressure, along with detailed protocols for facility design, equipment upkeep, and personnel hygiene. Together, these measures guarantee that compounded medications remain sterile, consistent, and thoroughly documented.
To help meet these rigorous requirements, 503Pharma offers practical resources, templates, and expert guidance tailored to USP <797> and FDA cGMP compliance.
Download Now
