USP 797 Immediate-Use Rules Explained
Apr 7, 2025
Introduction
The United States Pharmacopeia (USP) General Chapter <797>, titled "Pharmaceutical Compounding – Sterile Preparations," sets essential standards for preparing compounded sterile preparations (CSPs) to minimize risks to patients, such as microbial contamination, variability, and errors. Effective November 1, 2023, the revised chapter introduced significant updates, shifting from risk-based levels (low, medium, high) to a category system focused on environmental controls, beyond-use dates (BUDs), and testing.
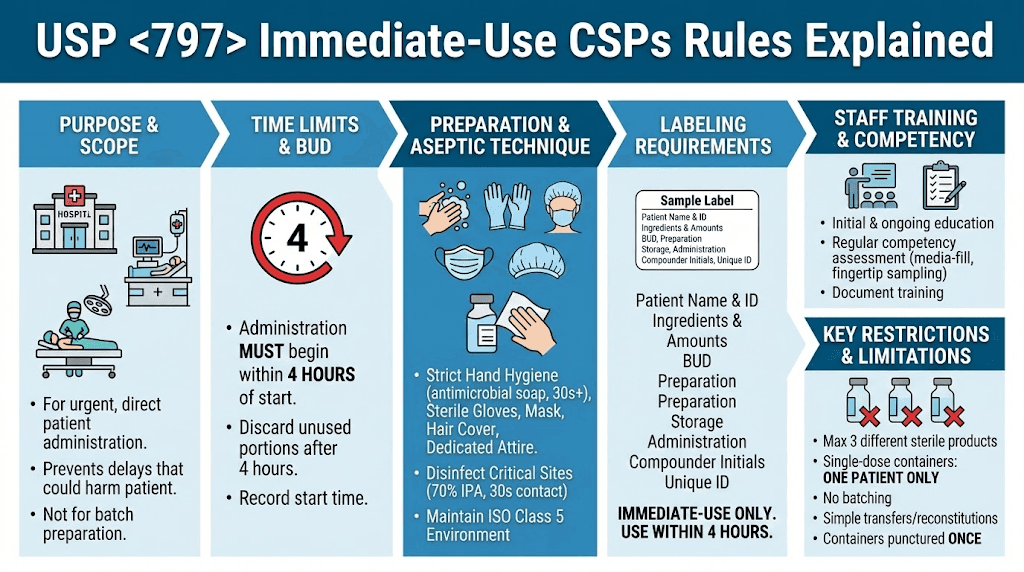
This post focuses on immediate-use CSPs, which are critical for urgent or emergency situations where delays could harm patients. These provisions allow for simplified preparation outside full cleanroom setups under strict conditions, emphasizing aseptic techniques to prioritize patient safety and compliance. We'll cover key definitions, requirements, and implementation strategies to help compounding pharmacies align with these guidelines.
Definitions
Compounded Sterile Preparation (CSP): A sterile drug product prepared from sterile or nonsterile ingredients, or a combination, that must be sterile to prevent harm to patients. Excludes preparations like irrigations for mouth or nasal cavities unless for internal use.
Immediate-Use CSP: A CSP intended for direct and immediate administration to a single patient in urgent situations. It is exempt from many <797> requirements if specific criteria are met, such as involving no more than three different sterile products and starting administration within four hours of preparation start.
Aseptic Technique: Methods to minimize contamination, including hand hygiene, garbing, and handling in a way that avoids contact with nonsterile surfaces.
Beyond-Use Date (BUD): The date and time after which a CSP must not be used, stored, or transported. For immediate-use, this is effectively the four (4)-hour window to begin administration.
Proprietary Bag and Vial Systems: Pre-filled systems where docking and activation for immediate administration do not count as compounding if following manufacturer labeling.

Principles
The core principles of USP <797> emphasize patient safety through contamination prevention, accurate preparation, and quality assurance. For immediate-use CSPs:
Prioritize urgency while maintaining sterility to avoid delays in critical care, such as in emergencies, surgeries, or acute treatments.
Minimize microbial growth by limiting preparation time and complexity.
Rely on evidence-based compatibility data from approved labeling or studies.
Ensure all processes follow facility-specific standard operating procedures (SOPs) to reduce risks like mix-ups or particulate introduction.
These principles align with broader <797> goals, shifting focus from risk levels to verifiable controls and competencies.
Categories
The 2023 revision replaces the old risk-level system with three categories based on compounding environment, controls, and intended BUD:
Category | Description | Key Requirements | Typical Use |
|---|---|---|---|
Category 1 | Prepared in a primary engineering control (PEC) in an unclassified segregated compounding area (SCA) or better. | Basic aseptic techniques; no sterility testing required. | Short BUDs; simple preparations. |
Category 2 | Prepared in a cleanroom suite with ISO Class 5 PEC, ISO Class 7 buffer room, and ISO Class 8 anteroom. | Enhanced monitoring; sterility testing for longer BUDs. | Moderate complexity; extended storage. |
Category 3 | Same as Category 2 but with additional controls like more frequent monitoring and testing. | Sterility testing mandatory; higher personnel competency assessments. | High-volume or long-BUD preparations. |
Immediate-use CSPs are a separate provision, exempt from these categories if criteria are met (e.g., no batching, single-patient use). If not met, they default to Category 1 or higher.
Storage Conditions
Storage must preserve sterility and stability:
Controlled Room Temperature (CRT): 20°C–25°C.
Refrigerated: 2°C–8°C.
Frozen: -25°C to -10°C.
For immediate-use CSPs, no storage is intended; they must be administered promptly. If delayed beyond four hours, discard safely. All CSPs require monitoring (e.g., temperature logs) and protection from light, moisture, or extremes.
BUD Limits
BUDs are assigned based on category, starting components, testing, and storage. For immediate-use, the limit is four hours to start administration at CRT.
Category | Aseptically Processed (No Sterility Testing) | Aseptically Processed (With Sterility Testing) | Terminally Sterilized (With Sterility Testing) |
|---|---|---|---|
Category 1 | ≤12 hours (CRT); ≤24 hours (refrigerated) | N/A | N/A |
Category 2 | Up to 4 days (CRT); 10 days (refrigerated); 45 days (frozen) for low-risk equivalents | Up to 30 days (CRT); 45 days (refrigerated); 60 days (frozen) | Up to 45 days (CRT); 60 days (refrigerated); 90 days (frozen) |
Category 3 | N/A | Up to 60 days (CRT); 90 days (refrigerated); 120 days (frozen) | Up to 90 days (CRT); 120 days (refrigerated); 180 days (frozen) |
Note: BUDs for immediate-use are not extended; discard if not administered within four hours.
Requirements for Extensions and Testing
Extensions: Longer BUDs require Category 2 or 3 compliance, including sterility testing per USP <71>, stability data, and enhanced monitoring. For Category 3, pass all tests (e.g., endotoxin for parenterals).
Testing:
Sterility: Mandatory for Category 3; optional for Category 2 extensions.
Microbial Identification: If action levels exceeded in monitoring.
Competency: Media-fill tests, gloved fingertip sampling.
Immediate-use: No testing required if criteria met; focus on aseptic process validation through training.
Environmental Controls
ISO Classifications: PEC must be ISO Class 5; buffer rooms ISO Class 7; anterooms ISO Class 8.
Monitoring: Viable air sampling every six months (Category 1/2) or monthly (Category 3); surface sampling monthly (1/2) or weekly (3).
Cleaning: Daily with EPA-registered agents; monthly sporicidal for longer BUDs.
Immediate-use: Exempt from classified environments if simple (≤3 products, no hazardous drugs); otherwise, use ISO Class 5 or better.
Personnel Training, Qualifications, and Garbing
Training: Initial and ongoing in aseptic techniques, SOPs, and compatibility. Assessments include written tests, media-fills (every 6–12 months), and fingertip sampling.
Qualifications: Designated person oversees; all personnel demonstrate competency before compounding.
Garbing:
Wash hands for 30 seconds.
Don low-lint garments, hair covers, masks, shoe covers.
Use sterile gloves; alcohol disinfection.
For immediate-use: Training focused on assigned tasks; no full garbing if exempt, but maintain hygiene.
Compliance and Quality Assurance
Documentation: Master formulation records (MFR) for nonsterile or multi-patient CSPs; compounding records (CR) for each preparation; quality logs for monitoring and calibration.
Audits: Regular environmental monitoring, trend analysis, and corrective actions.
Labeling: Include patient info, ingredients, BUD/time, preparer initials, "Immediate-Use Only" if applicable.
Benefits: Reduces contamination risks, ensures consistent quality, enhances patient outcomes, and supports regulatory inspections, ultimately protecting public health.
Implementation Steps
Alignment with State Rules
While USP <797> is a national standard, state boards of pharmacy may adopt it fully, partially, or with additions. Always check your state board for specifics.
State | Example Variation/Requirement |
|---|---|
Washington | Requires initial and annual (every 12 months) competency assessments for hand hygiene, garbing, cleaning, and calculations; aligns with USP but adds state addendum for documentation. |
Texas | Updates rules for personnel, environment, process, cleaning, and BUD to match revised USP; may include state-specific penalties or inspections. |
Florida | Adopts USP as minimum standards for sterile products; allows practices exceeding USP for enhanced safety. |
California | References sterile compounding regulations; no explicit USP <797> mandate but requires additional licensing and inspections for compounding facilities. |
New York | Complies with federal laws (FDCA); no direct USP adoption but outsourcing facilities must meet similar sterile standards. |
Pharmacist Decision-Making
Pharmacists play a key role in determining immediate-use applicability. Follow this process outline:
Assess urgency: Confirm delay would harm the patient.
Evaluate criteria: Ensure ≤3 sterile products, single-patient, no hazardous drugs.
Verify compatibility: Use approved labeling or studies.
Prepare aseptically: Follow SOPs for hygiene and handling.
Document: Record preparation time, ingredients, and administration plan.
Monitor: If criteria not met, escalate to categorized compounding.
Training Resources and Programs
Effective training is crucial for compliance. Consider these examples, including our newly launched CompoundLearn (compoundlearn.com), a learning management system offering tailored online modules for compounding pharmacies.
Resource/Program | Description |
|---|---|
ASHP Compounding Resources | Offers certificates and curricula updated for USP <797>, including garbing and monitoring modules. |
CriticalPoint Virtual Training | Focuses on best practices for nonhazardous sterile compounding, with interactive sessions on USP requirements. |
ASA E-Learning Module | Targeted at clinicians for immediate-use sterile compounding, covering aseptic techniques and compliance. |
CompoundLearn (compoundlearn.com) | Newly launched LMS with online modules on BUD calculation, risk assessment, and USP compliance; ideal for pharmacies seeking practical, tailored content. |
Expanding Knowledge and Continuous Improvement
Stay ahead by regularly reviewing USP updates and engaging in continuous education. Use case studies to simulate immediate-use scenarios, and implement tools for BUD tracking and risk assessments. For expanding knowledge, we recommend CompoundLearn (compoundlearn.com), which provides case studies, interactive tools, and aids for practical implementation in compounding pharmacies. This platform supports ongoing improvement by offering customized content focused on patient safety and regulatory adherence.
FAQ
What qualifies as an immediate-use CSP? Preparations for urgent administration involving no more than three sterile products, for a single patient, with administration starting within four (4) hours.
Can immediate-use CSPs be prepared outside a cleanroom? Yes, if all exemption criteria are met; otherwise, they must follow Category 1 requirements.
How often must personnel be trained? Initial training with ongoing assessments every 6–12 months, including media-fills and fingertip sampling.
What if a state rule differs from USP <797>? Follow the stricter standard; consult your state board of pharmacy.
Are there extensions for immediate-use BUDs? No; the four-hour limit is fixed to minimize risks.
How does CompoundLearn help with compliance? It offers newly launched modules on USP topics, including BUDs and aseptic techniques, tailored for compounding teams.
This comprehensive guide underscores the importance of USP <797> in safeguarding patients through rigorous standards. For the most accurate and current information, always consult official USP sources, such as usp.org, and your state board of pharmacy.
Related posts
Free Download: Retatrutide Compounding Toolkit 2026
Download Now
